
NextSeq 550Dx Reagents
These sequencing reagent kits offer a simplified workflow and high data quality for in vitro diagnostic testing on the NextSeq550 Dx Instrument.
Kits, services, and software solutions
Regulated products for IVD assay diagnostics, development, and clinical research

High-throughput library prep automation
Automation methods for many of our library prep assays are available from leading automation companies. Our partners offer a range of automation platforms to address your lab's throughput needs.
Laboratory information management systems (LIMS)
Use a LIMS to automate workflows, track reagents and lots, integrate instruments, and manage samples and associated information.
An integrated on-instrument software for creating sequencing runs, monitoring run status, and analyzing data (for diagnostic mode only). If your NextSeq 550Dx instrument requires installation of an application to run on the Local Run Manager (LRM), please contact your local sales team to arrange installation.
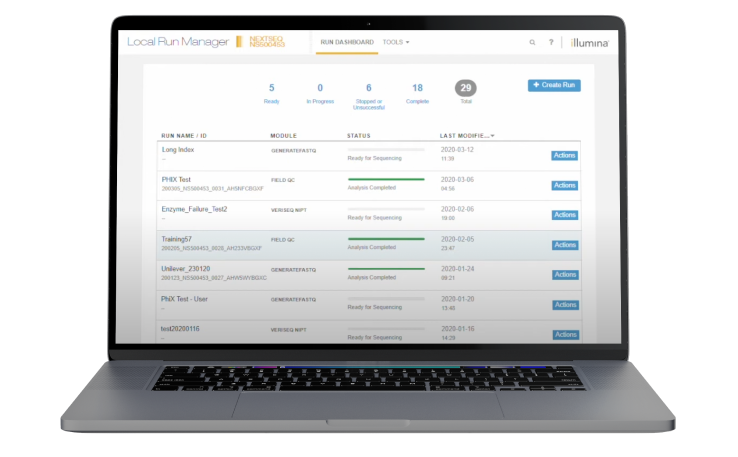
Illumina Run Manager (for diagnostic mode only) on the Illumina DRAGEN Server for NextSeq 550Dx Instruments enables user-friendly sequencing run setup and secondary data analysis to variant call format (VCF).
*Available only in select countries.
The Illumina genomics cloud-computing environment for NGS data analysis and management.
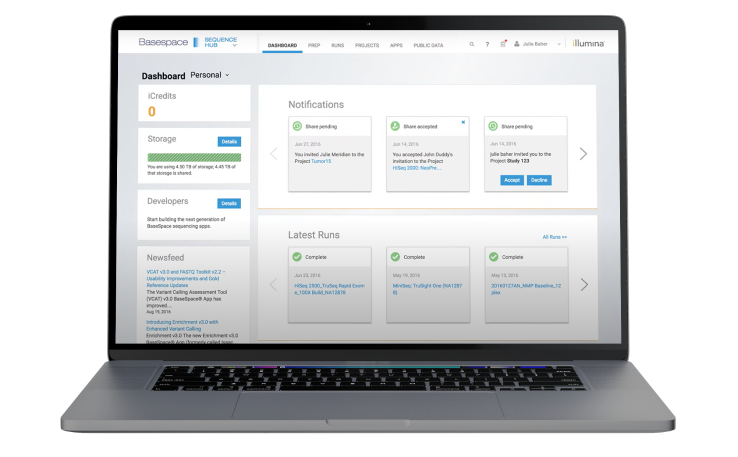
Interactive omics knowledge base that puts private omics data in biological context with highly curated public data.
Our variant annotation and analysis software tools can help researchers extract and report biological insights from large volumes of genomic data.
This remote instrument performance monitoring service helps you minimize unscheduled downtime for your Illumina sequencing systems.
Qualify Illumina laboratory instruments with professional services direct from the manufacturer.
Get hands-on NGS and microarray training from expert instructors. We also offer live or self-paced online courses and other educational resources.
Access a full suite of accurate, expedient solutions ranging from instrument service plans to qualification services and training.
The NextSeq 550Dx instrument is intended for targeted sequencing of DNA libraries from human genomic DNA extracted from peripheral whole blood or formalin-fixed, paraffin-embedded (FFPE) tissue when used with in vitro (IVD) diagnostic assays. The NextSeq 550Dx instrument is not intended for whole genome or de novo sequencing. The NextSeq 550Dx instrument is to be used with specific registered, certified, or approved in vitro diagnostic reagents and analytical software.
The NextSeq 550Dx instrument is intended for sequencing of DNA libraries when used with in vitro diagnostic assays. The NextSeq 550Dx instrument is to be used with specific registered, certified, or approved in vitro diagnostic reagents and analytical software.