RET Fusion Detection in Cancer
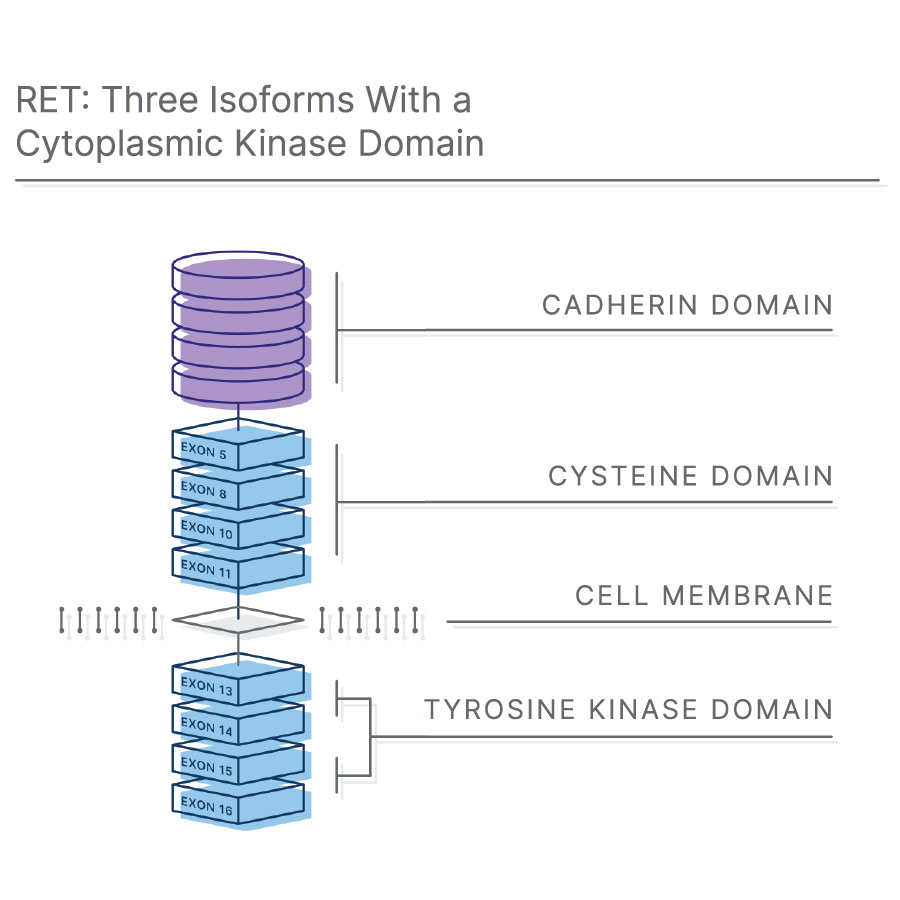
What is a RET fusion?
A RET fusion results in the activation of an oncogene that is implicated in the pathogenesis of multiple cancers. 1-4 RET is known to partner with at least 12 different genes, with KIF5B-RET being the most frequently observed RET fusion in NSCLC.5,6
The RET (Rearranged during Transfection) gene encodes a transmembrane receptor tyrosine kinase.7 RET acts as a receptor for Glial cell line-derived neurotrophic Family Ligands (GFL), a group of soluble neurotrophic factors that are highly important during embryogenesis and human development.8,9
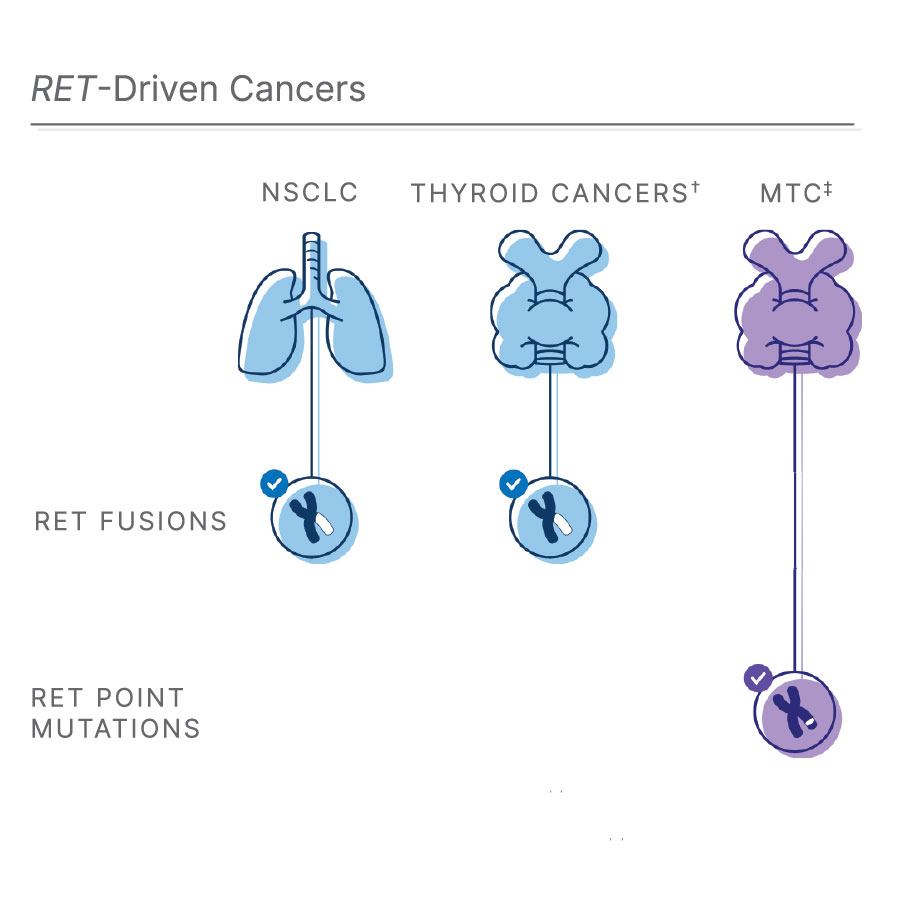
†RET fusions also occur in 10-20% of papillary thyroid cancers (PTC)1, 11, 12
‡Medullary thyroid cancer: RET point mutations affect most MTCs1, 3, 12
RET fusion implicated as growth driver in most common type of lung cancer
Lung cancer affects more than 2 million people each year and 85% of these cases occur as non-small cell lung cancer (NSCLC).10 Of those affected by NSCLC, about 2% are associated with RET fusion.1,8 In addition, RET fusions also occur in 10-20% of papillary thyroid cancers (PTC).1,11,12
While RET alterations are rare, new therapies make them highly actionable. With the FDA approval of the RET selective therapies RETEVMO® (selpercatinib) and GAVRETO® (pralsetinib), RET is an essential biomarker to test for in patients with metastatic NSCLC and thyroid cancer. Additionally, with the pan-tumor approval of RETEVMO® (selpercatinib), the detection of RET fusions has expanded treatment options for patients with advanced or metastatic solid tumors. Thus, in order to apply innovative precision medicines, all actionable biomarkers must be tested.
Detecting rare biomarkers using comprehensive genomic profiling
In an example study, out of 10,000 patients, 37% had actionable alterations identified by CGP.14 Given the availability of targeted therapies for RET fusions, the need for molecular characterization within NSCLC has expanded even further.
With a DNA+RNA workflow, CGP enables the simultaneous assessment of the growing number of common and rare driver mutations in a single test. By assessing all biomarkers at once, you may increase chances of finding an actionable alteration.
Bring cancer into focus with CGP
Simultaneously assess multiple biomarkers in numerous tumor types with a single NGS assay.
Learn more about CGPLearn more about RET fusion

Testing approaches for RET alterations in NSCLC
The landscape of biomarkers in NSCLC is evolving, and testing for RET alterations is increasingly important as new therapies target RET altered tumors. Learn more from Jaclyn Hechtman, MD, in this webinar.

Targeted therapy for RET fusion
Novel highly selective RET targeted agents have been tested in RET driven NSCLC, advancing targeted treatments. Dig deeper into these discoveries in this infographic.

Patient Success Story: Beating Stage IV Lung Cancer
AJ Patel had six months to live. Eight years later, he tells us how biomarker testing changed everything.
References
- Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin Cancer Res. 2017;23(8):1988-1997. doi:10.1158/1078-0432.CCR-16-1679
- Drilon A, Hu ZI, Lai GGY, Tan DSW. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15(3):150. doi:10.1038/nrclinonc.2017.188
- Pietrantonio F, Di Nicolantonio F, Schrock AB, et al. RET fusions in a small subset of advanced colorectal cancers at risk of being neglected. Ann Oncol. 2018;29(6):1394-1401. doi:10.1093/annonc/mdy090
- Su X, He C, Ma J, et al. RET/PTC Rearrangements Are Associated with Elevated Postoperative TSH Levels and Multifocal Lesions in Papillary Thyroid Cancer without Concomitant Thyroid Benign Disease. PLoS One. 2016;11(11):e0165596. Published 2016 Nov 1. doi:10.1371/journal.pone.0165596
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378-381. Published 2012 Feb 12. doi:10.1038/nm.2658
- Lipson D, Capelletti M, Yelensky R, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18(3):382-384. Published 2012 Feb 12. doi:10.1038/nm.2673
- Ibáñez CF. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol. 2013;5(2):a009134. doi:10.1101/cshperspect.a009134
- Wang X. Structural studies of GDNF family ligands with their receptors-Insights into ligand recognition and activation of receptor tyrosine kinase RET. Biochem Biophys Acta. 2013;1834:2205-2212
- de Graaff E, Srinivas S, Kilkenny C, et al. Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev. 2001;15(18):2433‐2444. doi:10.1101/gad.205001
- International Agency on Cancer. World Health Organization. Global Cancer Observatory website. Accessed June 24, 2020. https://gco.iarc.fr/
- Mulligan LM. RET revisited: expanding the oncogenic portfolio. Nat Rev Cancer. 2014;14(3):173-186. doi:10.1038/nrc3680
- Li AY, McCusker MG, Russo A, et al. RET fusions in solid tumors. Cancer Treat Rev. 2019;81:101911. doi:10.1016/j.ctrv.2019.101911
- Gautschi, O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: results from the global, multicenter RET registry. J Clin Oncol. 2017;35:1403-1410. doi:10.1200/JCO.2016.70.9352
- Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients [published correction appears in Nat Med. 2017 Aug 4;23 (8):1004]. Nat Med. 2017;23(6):703-713. doi:10.1038/nm.4333