A Genomic Solution for Noninvasive Prenatal Screening
NIPT (noninvasive prenatal testing) sensitivity and specificity are important considerations in choosing a test. The Verifi Prenatal Test is a highly accurate, noninvasive test that screens for aneuploidy of chromosomes 21, 18, and 13. Additional screening is available for sex chromosome aneuploidies, select microdeletions, and all autosomal trisomies in singleton pregnancies.
In twin pregnancies, screening for aneuploidy in chromosomes 21, 18, and 13 and the option to screen for the absence of the Y chromosome is available. Results are reported approximately 3–5 days after the sample is received. Depending on demand, the time to report may vary. Test performance metrics, including NIPT sensitivity, positive predictive values (PPV), and specificity data, are detailed below.
Are you a current customer?
Log In Now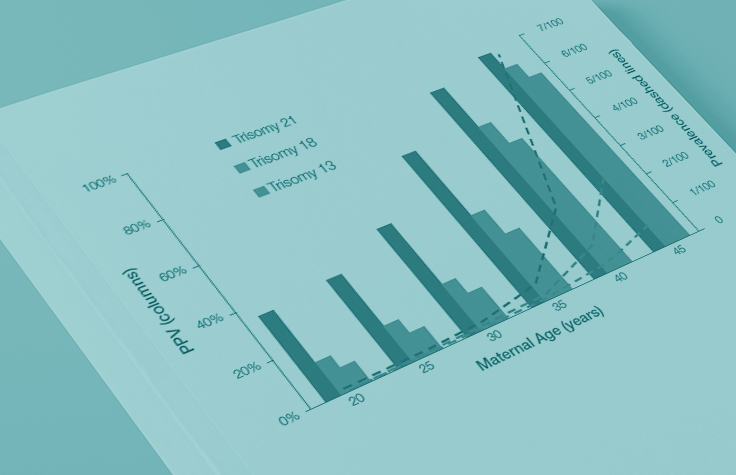
Publication: 85,000 NIPT Cases
Noninvasive prenatal testing in the general obstetric population: Clinical performance and counseling considerations in over 85,000 cases.
Test Performance Metrics for the Verifi Prenatal Test
Sensitivity and specificity for aneuploidy detected samples compared with published validation metrics
| CLIA Laboratory | Validation Study* | |||
|---|---|---|---|---|
| Condition | Observed Sensitivity† (Range)‡ | Observed Specificity† (Range)‡ | Sensitivity | Specificity |
| Trisomy 21 | 99.49% (98.66–99.53%) | 99.77% (98.92–99.91%) | 100% | 99.76% |
| Trisomy 18 | 97.23% (94.20–98.15%) | 99.69% (99.51–99.85%) | 97.37% | 99.57% |
| Trisomy 13 | 97.98% (95.56–98.87%) | 99.84% (99.77-99.93%) | 87.50% | 100% |
* Illumina. Analytical Validation of the verifi prenatal test: Enhanced Test Performance for Detecting Trisomies 21, 18, and 13 and the Option for Classification of Sex Chromosome Status. Illumina White Paper. 2012.
† Observed sensitivity and specificities were calculated using available outcome data with the cohort size adjusted for the proportion of positive cases with confirmed outcomes.
‡ The low end of the range was based on the assumption that all unreported outcomes are discordant, and high end of the range was based on the assumption that all unreported outcomes are concordant.
Performance Metrics Considerations After Screening
PPV based on performance metrics
Positive predictive value (PPV) refers to the proportion of positive test results that are truly positive. PPV is based on the sensitivity and specificity of the test and the prevalence of the condition in the population being tested. Because the prevalence of autosomal trisomies (eg trisomy 21) increases with maternal age, so do the PPVs.
For Verifi and Verifi Plus Prenatal Tests, if a woman receives an aneuploidy detected result for trisomy 21, trisomy 18, or trisomy 13 in a singleton pregnancy, the report will include a PPV based on the test’s sensitivity and specificity for the condition, maternal age, and gestational age.
Sample Test Report
| Chromosome | Results | PPV (%) |
|---|---|---|
| Chromosome 21 | POSITIVE: Aneuploidy detected Results consistent with pregnancy at increased risk for trisomy 21. |
95% |
| Chromosome 18 | NEGATIVE: No aneuploidy detected Results consistent with two copies of chromosome 18. |
|
| Chromosome 13 | NEGATIVE: No aneuploidy detected Results consistent with two copies of chromosome 13. |
Why Prevalence Matters for PPVs
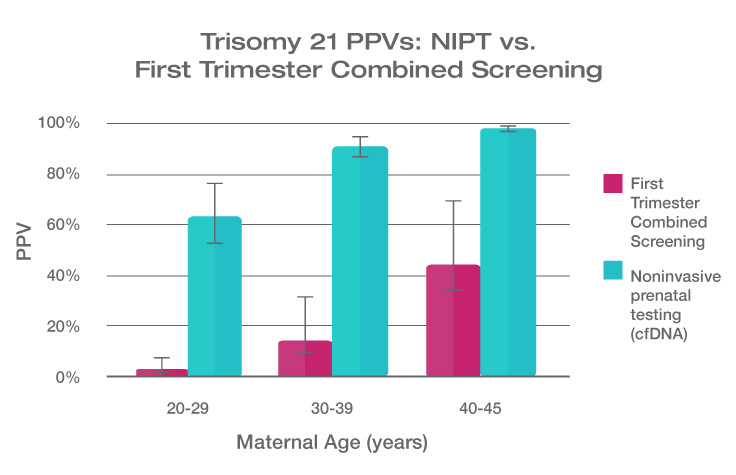
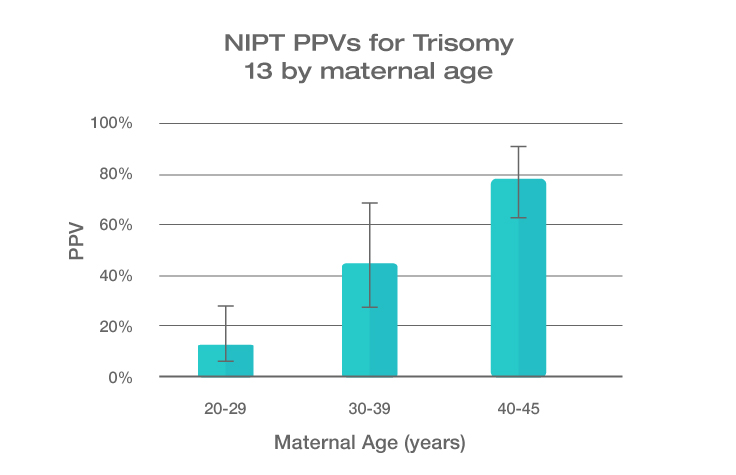
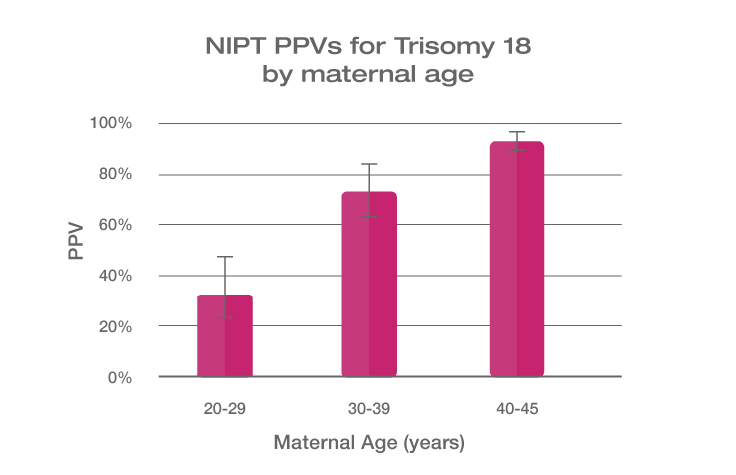
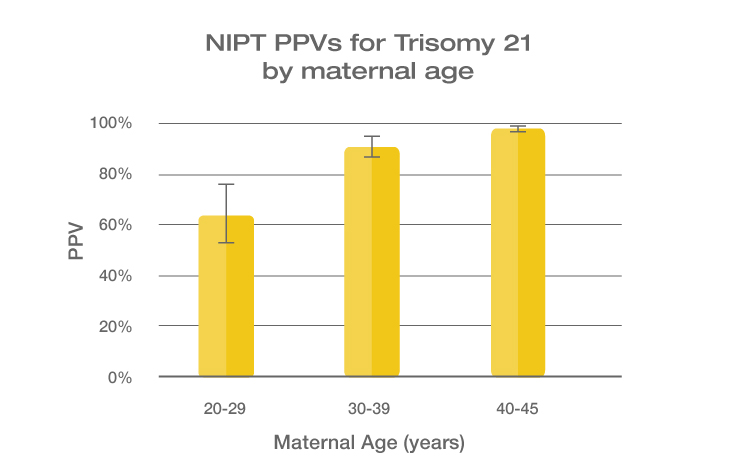
Because the prevalence of autosomal trisomies (eg, trisomy 21) increases with maternal age, so do the PPVs. Trisomy 21 is the most prevalent autosomal trisomy in live births. Thus, the PPVs are higher for trisomy 21 than for trisomies 18 and 13, which are less common, as seen in the graphs below.
The PPVs below are calculated based on age-related prevalence; the presence of other aneuploidy risk factors (eg, ultrasound abnormalities) would likely increase the PPVs over those shown here.
NIPT PPVs by maternal age
PPVs calculated using sensitivity and specificity data from a meta-analysis of 37 NIPT studies² and published estimates of aneuploidy prevalence at 10 weeks gestation for different maternal age groups.³⁻⁵ The potential PPV range, illustrated by the error bars, was calculated using 95% confidence intervals (CI) for sensitivity and specificity.
Assessing Test Failure Rate
TThe Verifi Prenatal test has a low failure rate of 0.6%.6 It uses next-generation sequencing to analyze cfDNA fragments across the whole genome, which has proven advantages over other NIPT methodologies such as targeted sequencing and array-based methods.
That’s important, and clinically relevant, because not only do test failures negatively impact patient care, they also adversely affect test metric parameters such as sensitivity, specificity, and PPV. By choosing the Verifi Prenatal Test, the clinical impact of failures can be reduced.4
The Illumina Difference
We’re committed to providing laboratories with comprehensive solutions and test options to improve human health. Published data shows that NGS with whole-genome sequencing (WGS) is the NIPT technology of choice when compared to targeted approaches.
With over 99.7% of NIPT samples in these studies run on Illumina NGS technology, we’re helping advance breakthroughs in prenatal screening.
Click on the below to view the samples run data table.
| Test (Company) | Current technology platform | Platform provider | Number of published samples | ||
|---|---|---|---|---|---|
| Illumina NGS | Ion Proton NGS | Affymetrix array | |||
| Bambni Test (Berry Genomics) | NGS | Illumina | 3,268 | 0 | 0 |
| MaterniT21 PLUS Test (Sequenom) | NGS | Illumina | 293,243 | 0 | 0 |
| NIFTY Test (BGI) | NGS | Illumina | 168,655 | 0 | 0 |
| Panorama Prenatal Screen (Natera) | NGS | Illumina | 55,077 | 0 | 0 |
| PrenaTest (LifeCodexx AG) | NGS | Illumina | 504 | 0 | 0 |
| Verifi Prenatal Test (Illumina) | NGS | Illumina | 113,561 | 0 | 0 |
| IONA Test (Premaltha) | NGS | Ion Proton | 0 | 684 | 0 |
| Harmony Prenatal Test (Arlosa)* | Array | Affymetrix | 44,313 | 0 | 1,677 |
| Total | 678,621 | 684 | 1,677 | ||
A PubMed search for "cell-free, DNA, prenatal," "noninvasive prenatal testing," and "noninvasive prenatal screening" was performed on November 30, 2015. All validation and clinical studies using unique samples were included, where a current clinical NIPT provider performed sample analysis. Case studies and studies published in a language other than English were excluded. A total of 59 published studies were surveyed. Data calculations on file. Illumina, Inc. 2015. NGS = next-generation sequencing; either whole-genome or targeted.
* In 2014, Ariosa switched from sequencing to arrays for clinical samples despite limited published data on this platform.
Test Failures May Lead to Invasive Procedures.†
Theoretical example of the number of invasive procedures requested due to NIPT failure and false positive rates of the assays. Failure rates include assay failures and samples rejected due to low fetal fraction. Assay failure rate for the Harmony test is based on NGS studies and may not be consistent with actual test results achieved using the array-based Harmony Test currently in use.3-7,9,10
† Affected pregnancies with a screening test failure were excluded from the number of detected T21.
Offering the Verifi Prenatal Test in Your Lab
Interested in becoming a select national laboratory partner?
Contact a Sales Rep
NIPT Sendout for Labs
Additional Resources

Learn more about the Verifi Prenatal Test
Get details about the Verifi Prenatal Test in one easy-to-read handout, which includes information about test options and performance data, as well as a sample report.

Learn more about the Verifi Plus Prenatal Test
Get the essential information about the broad range of the Verifi Plus Prenatal Test, including specifics on chromosomal aneuploidies identified, and read a sample report.

Choosing the Verifi Plus Prenatal Test
Gain deeper insight into the Verifi Plus Prenatal Test in this brochure, which discusses microdeletions, specific conditions and incidence rates, and offers clinical evidence.
References
- Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799-808.
- Ryan A, Hunkapiller N, Banjevic M, et al. Validation of an enhanced version of a single-nucleotide polymorphism-based noninvasive prenatal test for detection of fetal aneuploidies. Fetal Diagn Ther. 2016;40(3):219-223.
- McCullough RM, Almasri EA, Guan X, et al. Non-invasive prenatal chromosomal aneuploidy testing--clinical experience: 100,000 clinical samples. PLoS One. 2014;9(10):e109173.
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015: 372(17): 1589-97.
- Dar P, Curnow KJ, Gross SJ, et al. Clinical experience and follow-up with large scale single-nucleotide polymorphism-based noninvasive prenatal aneuploidy testing. Am J Obstet Gynecol. 2014: 211(5): 527.e1-527.e17.
- Data on file. Illumina, Inc 2022.
Verifi™ and Verifi™ Plus were developed by, and their performance characteristics were determined by Verinata Health, Inc. (VHI) a wholly owned subsidiary of Illumina, Inc. The VHI laboratory is CAP-accredited and certified under the Clinical Laboratory Improvement Amendments (CLIA) as qualified to perform high complexity clinical laboratory testing. They have not been cleared or approved by the U.S. Food and Drug Administration.