
NovaSeq X Series ordering
Advanced chemistry, optics, and informatics combine to deliver exceptional sequencing speed and data quality, outstanding throughput, and scalability.
Enable CGP with a large pan-cancer panel covering all main variant classes plus key biomarkers (TMB, MSI, and HRD) from FFPE tissue.
Assay time
Hands-on time
Input quantity
TruSight Oncology 500 v2 automation kits and methods available now. Contact an Illumina representative for more information.
TruSight Oncology 500 v2 is a pan-cancer next-generation sequencing (NGS) assay that enables in-house comprehensive genomic profiling (CGP) from formalin-fixed, paraffin-embedded (FFPE) tissue for oncology research.
HRD is a genomic signature linked to a cell’s inability to repair double-stranded DNA breaks, leading to genomic instability and tumorigenesis. HRD analysis is now integrated into the standard workflow, providing BRCA1/2 variant detection and genomic instability score (GIS) with no added time or complexity.
Illumina has partnered with leading liquid-handling manufacturers to produce fully automated workflows that can reduce hand-on time by ~50%. This improves efficiency while achieving the same high-quality results produced by manual protocols.
*Illumina Connected Insights supports user-defined tertiary analysis through API calls to third-party knowledge sources.
Identify disease-relevant biomarkers for oncology research with the TruSight Oncology 500 product line.
Analyze circulating tumor DNA (ctDNA) in blood plasma via liquid biopsy with similar DNA panel content as TruSight Oncology 500 v2.
| Assay time | 3–4 days from sample input to final results |
|---|---|
| Automation capability | Liquid handling robot(s) |
| Automation details | Explore available automation methods |
| Cancer type | Pan-cancer, Solid tumor |
| Content specifications |
Targeted sequencing of DNA from 523 genes and RNA from 55 genes for a total of 1.94 Mb panel size. MSI and TMB measurement included. The included HRD panel † includes coverage of ~25K SNPs to assess homologous recombination deficiency through a comprehensive genomic instability score (LOH+TAI+LST) powered by Myriad Genetics. †TruSight Oncology 500 v2 is not available for sale in Japan |
| Description | Enables comprehensive genomic profiling of solid tumors using DNA and RNA from FFPE tissue. Provides variant detection and biomarker assessment from a single sample for broad tumor characterization. |
| Hands-on time |
~3.25 hrs for automated workflow ~5-7 hrs for manual workflow |
| Input quantity | 30 ng DNA (as low as 10 ng), 40 ng RNA (as low as 20 ng) |
| Instruments | NextSeq 550 System, NextSeq 2000 System, NextSeq 1000 System, NextSeq 550Dx in Research Mode, NovaSeq X System, NovaSeq 6000Dx in Research Mode, NovaSeq 6000 System, NovaSeq X Plus System |
| Method | Targeted DNA sequencing, Targeted RNA sequencing, Target enrichment |
| Multiplexing |
NextSeq 550/Dx: 8 samples/run. NexSeq 1000 and 2000: P2 flow cell 8 samples, P3 flow cell 24 samples, P4 flow cell 36 samples. NovaSeq 6000/Dx: SP flow cell 16 samples, S1 flow cell 32 samples, S2 flow cell 72 samples, S4 flow cell 192 samples. NovaSeq X/X+: 1.5 B 32 samples, 10B 192 samples, 25B 480 samples. |
| Nucleic acid type | DNA, RNA |
| Sample throughput | 8–960 samples per run |
| Specialized sample types | FFPE tissue |
| Species category | Human |
| Technology | Sequencing |
| Variant class | Gene fusions, Loss of heterozygosity (LOH), Somatic variants, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs), Tumor mutational burden (TMB), Genomic instability score (GIS), Microsatellite instability (MSI), Novel transcripts, Single nucleotide polymorphisms (SNPs), Structural variants |
To run TruSight Oncology 500 v2 you need:
To analyze with the DRAGEN variant calling pipeline you need:
*NovaSeq 6000Dx paired DRAGEN server is enabled for TruSight Oncology 500 v2 local variant calling. All other instruments require a separate, stand-alone DRAGEN server for on-premise secondary analysis.
TruSight Oncology 500 v2 enables comprehensive genomic profiling of FFPE tumor samples to assess a wide range of biomarkers using less sample, providing more informative results than single-gene or small panel assays.
TruSight Oncology 500 v2
| Instrument | Recommended number of samples | Read length |
|---|---|---|
| NextSeq 500 System, NextSeq 550Dx in Research Mode | 8 samples per run (high output flow cell), 100M paired-end reads, 3,500× coverage |
2 × 101 bp |
| NextSeq 1000 System, NextSeq 2000 System | 8 samples per run (P2 flow cell), 100M paired-end reads, 3,500× coverage 24 samples per run (P3 flow cell), 100M paired-end reads, 3,500× coverage 36 samples per run (P4 flow cell), 100M paired-end reads, 3,500× coverage |
2 × 101 bp |
| NovaSeq X System | 32 samples per run (1.5B flow cell), 100M paired-end reads, 3,500× coverage 192 samples per run (10B flow cell), 100M paired-end reads, 3,500× coverage 480 samples per run (25B flow cell), 100M paired-end reads, 3,500× coverage
|
2 × 101 bp |
| NovaSeq 6000 System, NovaSeq 6000Dx in Research Mode | 16 samples per run (SP flow cell), 100M paired-end reads, 3,500x coverage 32 samples per run (S1 flow cell), 100M paired-end reads, 3,500x coverage 72 samples per run (S2 flow cell), 100M paired-end reads, 3,500x coverage 192 samples per run (S4 flow cell), 100M paired-end reads, 3,500x coverage |
2 × 101 bp |
Pathology and clinical cancer research
Our clinical cancer research solutions deliver accurate genomic information, and enable labs to analyze multiple genes in a single test.
Next-generation sequencing enables immuno-oncology researchers to gain insights into immunotherapy response factors and tumor immune evasion mechanisms.
NGS-based cancer sequencing methods provide more information in less time compared to single-gene and array-based approaches.
| TruSight Oncology 500 v2 | TruSight Oncology 500 High-Throughput | TruSight Oncology 500 ctDNA v2 | |
|---|---|---|---|
| Assay time | 3–4 days from sample input to final results | 4–5 days from sample to results | 2.5–4 days from purified nucleic acid to variant results |
| Automation capability | Liquid handling robot(s) | Liquid handling robot(s) | Liquid handling robot(s) |
| Automation details | Explore available automation methods | Explore available automation methods | Explore available automation methods |
| Cancer type | Pan-cancer, Solid tumor | Pan-cancer, Solid tumor | Pan-cancer, Solid tumor |
| Content specifications |
Targeted sequencing of DNA from 523 genes and RNA from 55 genes for a total of 1.94 Mb panel size. MSI and TMB measurement included. The included HRD panel † includes coverage of ~25K SNPs to assess homologous recombination deficiency through a comprehensive genomic instability score (LOH+TAI+LST) powered by Myriad Genetics. †TruSight Oncology 500 v2 is not available for sale in Japan |
Targeted sequencing of DNA from 523 genes of interest and RNA from 55 genes, for a total of 1.94 Mb panel size. MSI and TMB measurement included. | Targeted sequencing of DNA for a total of 1.94 Mb panel size: 523 genes for small variants, 59 genes for CNVs, 23 genes for gene rearrangements, bMSI (> 2300 loci), bTMB (1.94 Mb). |
| Description | Enables comprehensive genomic profiling of solid tumors using DNA and RNA from FFPE tissue. Provides variant detection and biomarker assessment from a single sample for broad tumor characterization. | A high-throughput comprehensive NGS assay to identify key biomarkers in guidelines and >1K clinical trials from a streamlined workflow using the NextSeq 1000 System, NextSeq 2000 System, NovaSeq 6000 System, NovaSeq 6000Dx instrument (in Research Mode), or NovaSeq X Series. Includes coverage of immuno-oncology biomarkers TMB and MSI. | Provides a noninvasive research method for comprehensive genomic profiling of liquid biopsy samples (ctDNA from blood plasma). This liquid biopsy approach provides insights about intra- and inter-tumor heterogeneity using a minimally invasive sample collection approach to complement tissue-based CGP. |
| Hands-on time |
~3.25 hrs for automated workflow ~5-7 hrs for manual workflow |
~2.5 hr for automated workflow ~10.5 hr for manual workflow |
~1.5 hr for automated workflow ~2.5 hr for manual workflow |
| Input quantity | 30 ng DNA (as low as 10 ng), 40 ng RNA (as low as 20 ng) | 40 ng DNA and/or 40–80 ng RNA | 20 ng cfDNA (4 ml of plasma) |
| Instruments | NextSeq 550 System, NextSeq 2000 System, NextSeq 1000 System, NextSeq 550Dx in Research Mode, NovaSeq X System, NovaSeq 6000Dx in Research Mode, NovaSeq 6000 System, NovaSeq X Plus System | NextSeq 2000 System, NextSeq 1000 System, NovaSeq X System, NovaSeq 6000Dx in Research Mode, NovaSeq 6000 System, NovaSeq X Plus System | NextSeq 2000 System, NovaSeq X System, NovaSeq 6000Dx in Research Mode, NovaSeq 6000 System, NovaSeq X Plus System |
| Method | Targeted DNA sequencing, Targeted RNA sequencing, Target enrichment | Targeted DNA sequencing, Targeted RNA sequencing, Target enrichment | Targeted DNA sequencing, Target enrichment |
| Multiplexing |
NextSeq 550/Dx: 8 samples/run. NexSeq 1000 and 2000: P2 flow cell 8 samples, P3 flow cell 24 samples, P4 flow cell 36 samples. NovaSeq 6000/Dx: SP flow cell 16 samples, S1 flow cell 32 samples, S2 flow cell 72 samples, S4 flow cell 192 samples. NovaSeq X/X+: 1.5 B 32 samples, 10B 192 samples, 25B 480 samples. |
NextSeq 1000 and 2000: P2 flow cell 8 samples, P3 flow cell 24 samples, P4 flow cell 36 samples. NovaSeq 6000/Dx: SP flow cell 16 samples, S1 flow cell 32 samples, S2 flow cell 72 samples, S4 flow cell 192 samples. NovaSeq X Series*: 1.5B cartridge 32 samples, 10B cartridge 192 samples, 25B 480 samples. |
NextSeq 2000 System: 4 samples on P4 flow cell. NovaSeq 6000 System: 4 samples on S1 flow cell, 8 samples on S2 flow cell, 24 samples on S4 flow cell. NovaSeq X Series: 4 samples on 1.5B flow cell, 24 samples on 10B flow cell, 64 samples on 25B flow cell. |
| Nucleic acid type | DNA, RNA | DNA, RNA | DNA |
| Sample throughput | 8–960 samples per run | NextSeq 1000 and 2000: 8–36 samples/run. NovaSeq 6000/Dx: 16-192 samples/run. NovaSeq X: 32–480 samples/run. NovaSeq X Plus: 32-960 samples/run. | 4-128 samples/run |
| Specialized sample types | FFPE tissue | FFPE tissue | Circulating tumor DNA, Blood |
| Species category | Human | Human | Human |
| Technology | Sequencing | Sequencing | Sequencing |
| Variant class | Gene fusions, Loss of heterozygosity (LOH), Somatic variants, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs), Tumor mutational burden (TMB), Genomic instability score (GIS), Microsatellite instability (MSI), Novel transcripts, Single nucleotide polymorphisms (SNPs), Structural variants | Gene fusions, Somatic variants, Novel transcripts, Structural variants, Transcript variants, Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs) | Single nucleotide variants (SNVs), Insertions-deletions (indels), Copy number variants (CNVs), Blood microsatellite instability (bMSI), Blood tumor mutational burden (bTMB), Gene rearrangements |
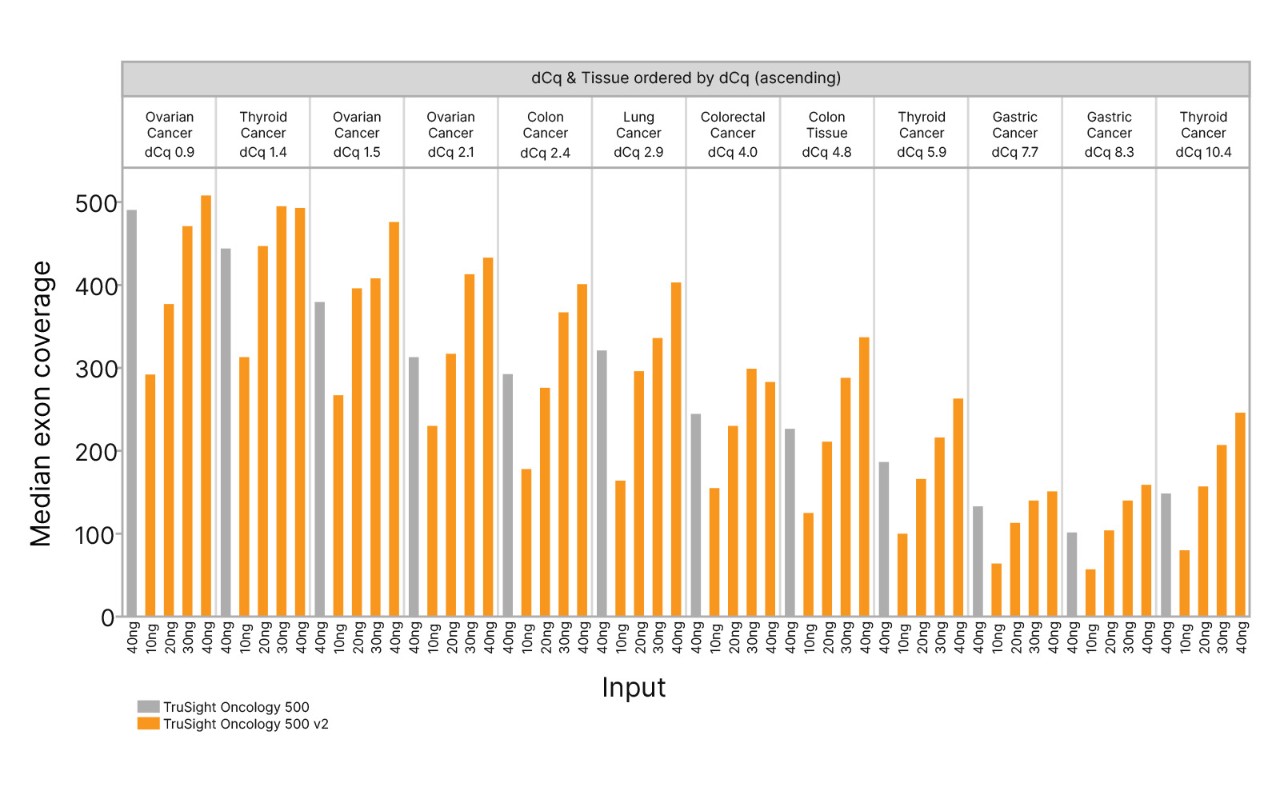

TruSight Oncology 500 v2 showed improved median exon coverage compared to the original TruSight Oncology 500 across 13 cancer types for 10–40 ng DNA input. Improved exon coverage was observed across most conditions, particularly for lower input levels.
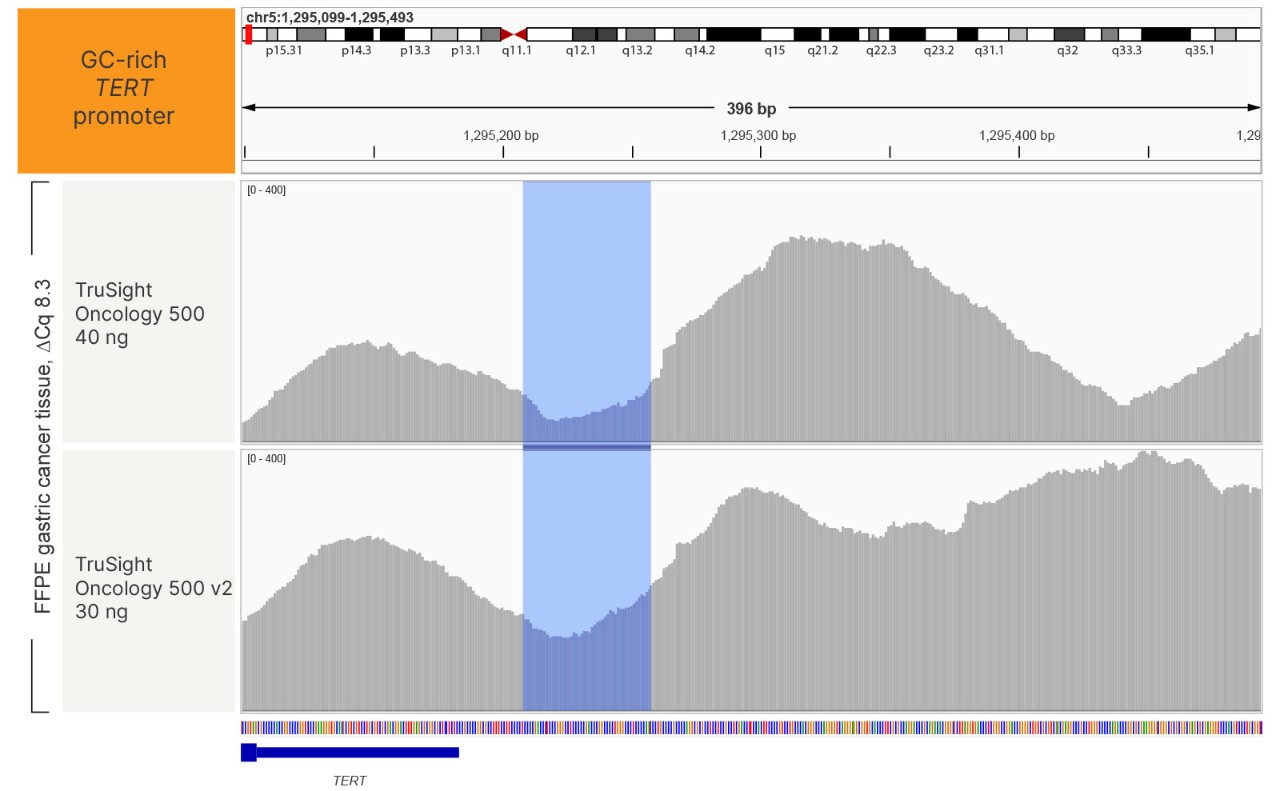

TruSight Oncology 500 v2 has improved coverage of GC-rich regions, such as the TERT promoter, which are often difficult to amplify by PCR.
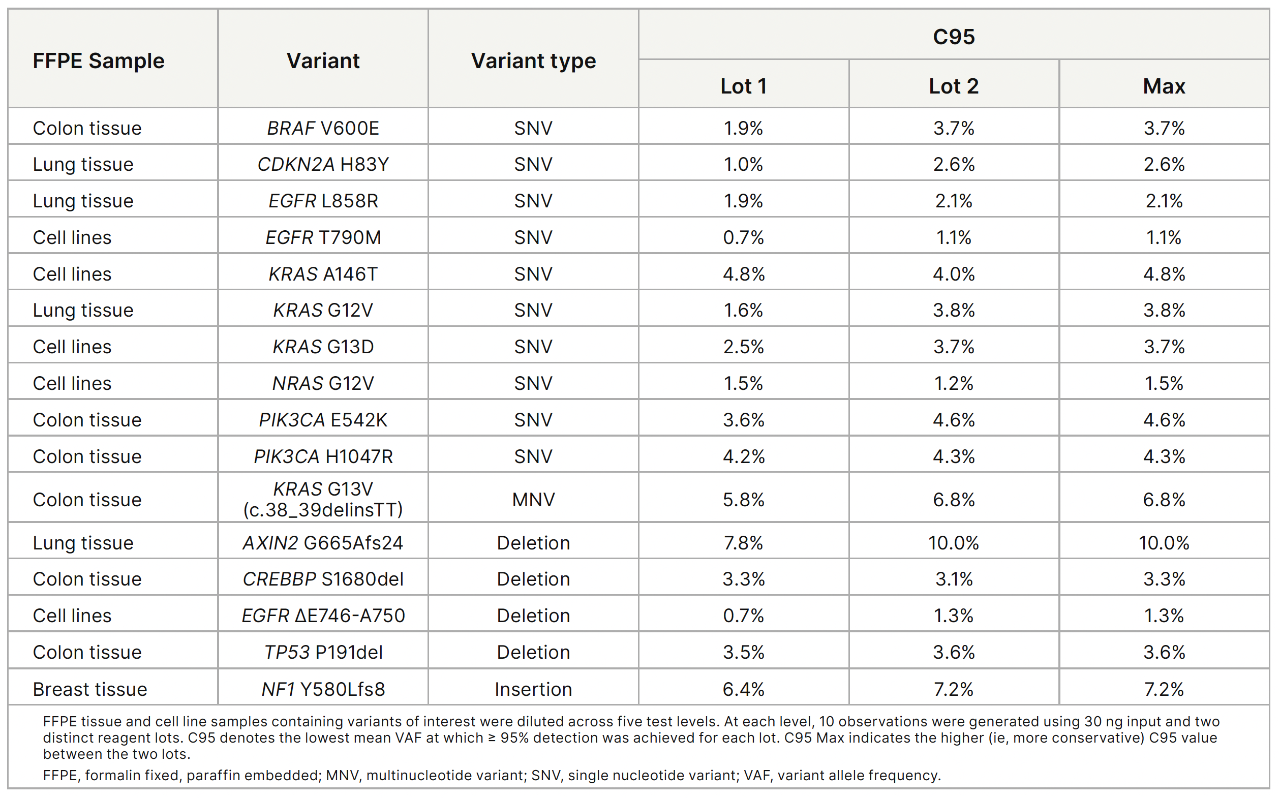

TruSight Oncology 500 v2 showed accurate detection of single nucleotide variants (SNVs), multinucleotide variants (MNVs), and indels across input levels and reagent lots.

TruSight Oncology 500 v2 demonstrated reliable copy number variant (CNV) detection, as indicated by mean fold change. Consistent performance was observed for 10–40 ng DNA input.
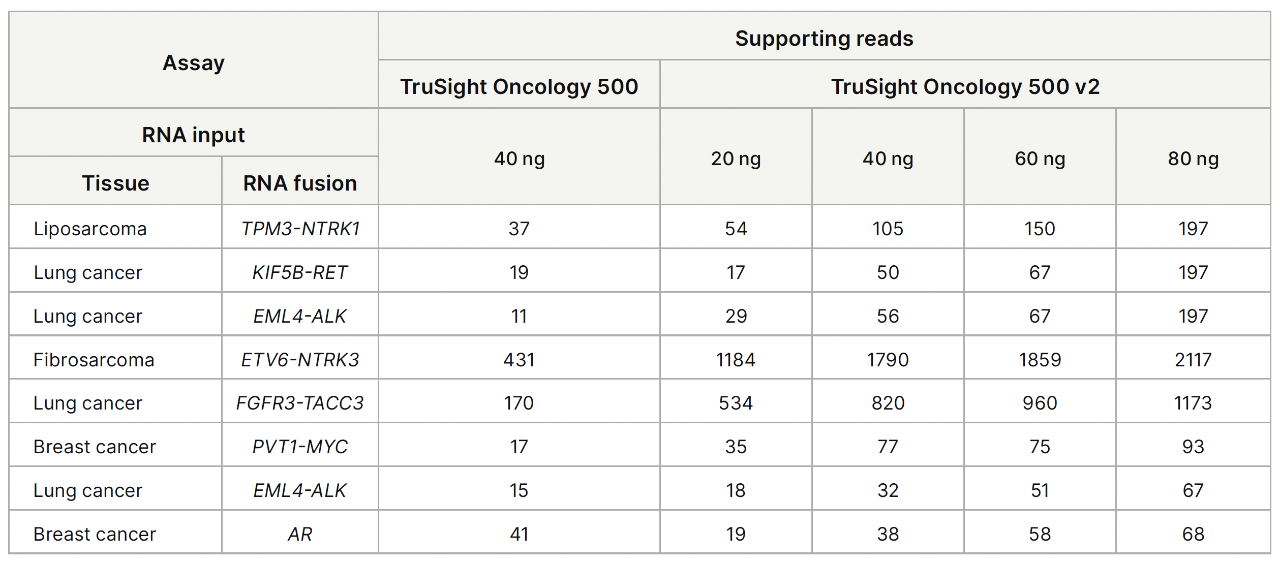

TruSight Oncology 500 v2 showed sensitive gene fusion and splice variant detection across 20–80 ng RNA input.
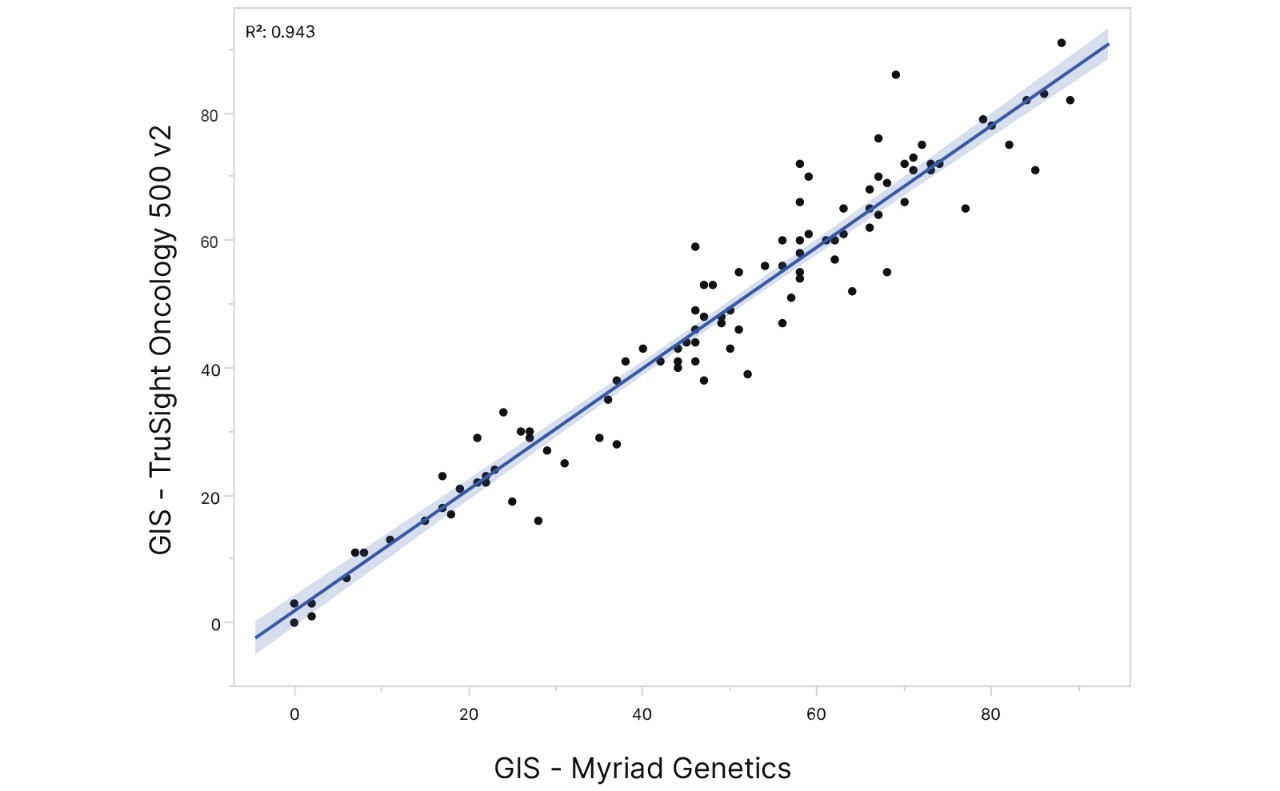

GIS results from TruSight Oncology 500 v2 using 30 ng DNA input showed high concordance to a reference test across 102 FFPE tissue samples.
Pan-cancer: BRAF, NTRK1, NTRK2, NTRK3, RET, TMB, MSI
| Genes with biomarkers of significancea | ||||||||
|---|---|---|---|---|---|---|---|---|
| AKT1 | BRCA1 | BRCA2 | ESR1 | PIK3CA | PTEN | |||
| KRAS | NRAS | POLD1 | POLE | |||||
| ALK | BRAF | EGFR | ERBB2 | KRAS | MET | |||
| RET | ROS1 | |||||||
| KIT | NRAS | |||||||
| BRCA1 | BRCA2 | HRD | ||||||
| ATM | ATR | BARD1 | BRCA1 | BRCA2 | BRIP1 | |||
| CDK12 | CHEK1 | CHEK2 | FANCL | MRE11A | NBN | |||
| PALB2 | RAD51B | RAD51C | RAD51D | RAD54L | ||||
| POLE | ||||||||
The genes and biomarkers listed in this table are a subset of all genes included in the panel. See the TruSight Oncology 500 data sheet for the full gene list.
References:
How TruSight Oncology 500 v2 is helping to advance cancer research at the Catalan Institute of Oncology
Learn how researchers at the Catalan Institute of Oncology are using TruSight Oncology 500 v2 to streamline their workflow and accelerate their research. In this video, scientists from the institute talk about the improved assay and share how faster results help to drive discovery.
TruSight Oncology 500 v2 DNA/RNA Kit (24 samples)
20130527
Includes reagents for preparing and enriching up to 24 DNA and RNA samples. Purchase sequencing reagents and indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Kit (48 samples)
20130528
Includes reagents for preparing and enriching up to 48 DNA samples. Purchase sequencing reagents and indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Kit, For Use with NextSeq 550 (24 samples)
20130536
Includes reagents for preparing and enriching up to 24 DNA and RNA samples, and NextSeq 500/550 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Kit, For Use with NextSeq 550 (48 samples)
20130537
Includes reagents for preparing and enriching up to 48 DNA samples, and NextSeq 500/550 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Kit, For Use with NextSeq 1000/2000 P2 (24 samples)
20138676
Includes reagents for preparing and enriching up to 24 DNA and RNA samples, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Kit, For Use with NextSeq 1000/2000 P2 (48 samples)
20138677
Includes reagents for preparing and enriching up to 48 DNA samples, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Kit plus Velsera interpretation report (24 samples)
20138680
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and data interpretation reports using Velsera Clinical Genomics Workspace. Purchase sequencing reagents and indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Kit plus Velsera interpretation report (48 samples)
20138681
Includes reagents for preparing and enriching up to 48 DNA samples and data interpretation reports using Velsera Clinical Genomics Workspace. Purchase sequencing reagents and indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Kit plus Velsera interpretation report, For Use with NextSeq 550 (24 samples)
20138686
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Kit plus Velsera interpretation report, For Use with NextSeq 550 (48 samples)
20138687
Includes reagents for preparing and enriching up to 48 DNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Kit plus Velsera interpretation report, For Use with NextSeq 1000/2000 P2 (24 samples)
20138690
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Kit plus Velsera interpretation report, For Use with NextSeq 1000/2000 P2 (48 samples)
20138692
Includes reagents for preparing and enriching up to 48 DNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Kit plus Illumina Connected Insights Software (24 samples)
20138695
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and data interpretation reports using Illumina Connected Insights. Purchase sequencing reagents and indexes separately.
TruSight Oncology 500 v2 DNA Kit plus Illumina Connected Insights Software (48 samples)
20138696
Includes reagents for preparing and enriching up to 48 DNA samples and data interpretation reports using Illumina Connected Insights. Purchase sequencing reagents and indexes separately.
TruSight Oncology 500 v2 DNA/RNA Kit plus Illumina Connected Insights Software, For Use with NextSeq 550 (24 samples)
20138775
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 500/550 sequencing reagents. Purchase indexes separately.
TruSight Oncology 500 v2 DNA Kit plus Illumina Connected Insights Software, For Use with NextSeq 550 (48 samples)
20138776
Includes reagents for preparing and enriching up to 48 DNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 500/550 sequencing reagents. Purchase indexes separately.
TruSight Oncology 500 v2 DNA/RNA Kit plus Illumina Connected Insights Software, For Use with NextSeq 1000/2000 P2 (24 samples)
20138779
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately.
TruSight Oncology 500 v2 DNA Kit plus Illumina Connected Insights Software, For Use with NextSeq 1000/2000 P2 (48 samples)
20138780
Includes reagents for preparing and enriching up to 48 DNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately.
TruSight Oncology 500 v2 DNA/RNA Automation Kit (32 samples)
20130529
Includes reagents for preparing and enriching up to 32 DNA and RNA samples. Purchase sequencing reagents and indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Automation Kit (64 samples)
20130530
Includes reagents for preparing and enriching up to 64 DNA samples. Purchase sequencing reagents and indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit (96 samples)
20130532
Includes reagents for preparing and enriching up to 96 DNA and RNA samples. Purchase sequencing reagents and indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit, For Use with NextSeq 550 (32 samples)
20130542
Includes reagents for preparing and enriching up to 32 DNA and RNA samples, and NextSeq 500/550 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Automation Kit, For Use with NextSeq 550 (64 samples)
20130543
Includes reagents for preparing and enriching up to 64 DNA samples, and NextSeq 500/550 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit, For Use with NextSeq 1000/2000 P2 (32 samples)
20138678
Includes reagents for preparing and enriching up to 32 DNA and RNA samples, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Automation Kit, For Use with NextSeq 1000/2000 P2 (64 samples)
20138679
Includes reagents for preparing and enriching up to 64 DNA samples, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Velsera interpretation report (32 samples)
20138682
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and data interpretation reports using Velsera Clinical Genomics Workspace. Purchase sequencing reagents and indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Automation Kit plus Velsera interpretation report (64 samples)
20138683
Includes reagents for preparing and enriching up to 64 DNA samples and data interpretation reports using Velsera Clinical Genomics Workspace. Purchase sequencing reagents and indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Velsera interpretation report (96 samples)
20138685
Includes reagents for preparing and enriching up to 96 DNA and RNA samples and data interpretation reports using Velsera Clinical Genomics Workspace. Purchase sequencing reagents and indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Velsera interpretation report, For Use with NextSeq 550 (32 samples)
20138688
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Automation Kit plus Velsera interpretation report, For Use with NextSeq 550 (64 samples)
20138689
Includes reagents for preparing and enriching up to 64 DNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Velsera interpretation report, For Use with NextSeq 1000/2000 P2 (32 samples)
20138693
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA Automation Kit plus Velsera interpretation report, For Use with NextSeq 1000/2000 P2 (64 samples)
20138694
Includes reagents for preparing and enriching up to 64 DNA samples and data interpretation reports using Velsera Clinical Genomics Workspace, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Illumina Connected Insights Software (32 samples)
20138698
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and data interpretation reports using Illumina Connected Insights. Purchase sequencing reagents and indexes separately. Intended for use with automation.
TruSight Oncology 500 v2 DNA Automation Kit plus Illumina Connected Insights Software (64 samples)
20138773
Includes reagents for preparing and enriching up to 64 DNA and RNA samples and data interpretation reports using Illumina Connected Insights. Purchase sequencing reagents and indexes separately. Intended for use with automation.
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Illumina Connected Insights Software (96 samples)
20138774
Includes reagents for preparing and enriching up to 96 DNA and RNA samples and data interpretation reports using Illumina Connected Insights. Purchase sequencing reagents and indexes separately. Intended for use with automation.
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Illumina Connected Insights Software, For Use with NextSeq 550 (32 samples)
20138777
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 500/550 sequencing reagents. Purchase indexes separately. Intended for use with automation.
TruSight Oncology 500 v2 DNA Automation Kit plus Illumina Connected Insights Software, For Use with NextSeq 550 (64 samples)
20138778
Includes reagents for preparing and enriching up to 64 DNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 500/550 sequencing reagents. Purchase indexes separately. Intended for use with automation.
TruSight Oncology 500 v2 DNA/RNA Automation Kit plus Illumina Connected Insights Software, For Use with NextSeq 1000/2000 P2 (32 samples)
20138781
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately. Intended for use with automation.
TruSight Oncology 500 v2 DNA Automation Kit plus Illumina Connected Insights Software, For Use with NextSeq 1000/2000 P2 (64 samples)
20138782
Includes reagents for preparing and enriching up to 64 DNA samples and data interpretation reports using Illumina Connected Insights, and NextSeq 1000/2000 P2 sequencing reagents. Purchase indexes separately. Intended for use with automation.
Illumina® DNA/RNA UD Indexes Set A, Tagmentation (96 Indexes, 96 Samples)
20091654
Illumina® DNA/RNA UD Indexes Set A, Tagmentation (96 Indexes, 96 Samples) Includes 96, 10 bp indexes sufficient for labeling 96 samples. Purchase library prep and enrichment reagents and probe panels separately.
List Price:
Discounts:
Illumina® DNA/RNA UD Indexes Set B, Tagmentation (96 Indexes, 96 Samples)
20091656
Illumina® DNA/RNA UD Indexes Set B, Tagmentation (96 Indexes, 96 Samples) Includes 96, 10 bp indexes sufficient for labeling 96 samples. Purchase library prep and enrichment reagents and probe panels separately.
List Price:
Discounts:
Illumina® DNA/RNA UD Indexes Set C, Tagmentation (96 Indexes, 96 Samples)
20091658
Illumina® DNA/RNA UD Indexes Set C, Tagmentation (96 Indexes, 96 Samples) Includes 96, 10 bp indexes sufficient for labeling 96 samples. Purchase library prep and enrichment reagents and probe panels separately.
List Price:
Discounts:
Illumina® DNA/RNA UD Indexes Set D, Tagmentation (96 Indexes, 96 Samples)
20091660
Illumina® DNA/RNA UD Indexes Set D, Tagmentation (96 Indexes, 96 Samples) Includes 96, 10 bp indexes sufficient for labeling 96 samples. Purchase library prep and enrichment reagents and probe panels separately.
List Price:
Discounts:
Illumina® DNA/RNA UD Indexes v3, Set A, Auto (96 Indexes 96 samples)
20141196
Includes one box of 96 Illumina DNA/RNA UD v3 Indexes Set A for labeling 96 samples sufficient for automation
List Price:
Discounts:
Illumina® DNA/RNA UD Indexes v3, Set B, Auto (96 Indexes 96 samples)
20141197
Includes one box of 96 Illumina DNA/RNA UD v3 Indexes Set B for labeling 96 samples sufficient for automation
List Price:
Discounts:
Illumina® DNA/RNA UD Indexes v3, Set C, Auto (96 Indexes 96 samples)
20141198
Includes one box of 96 Illumina DNA/RNA UD v3 Indexes Set C for labeling 96 samples sufficient for automation
List Price:
Discounts:
Illumina® DNA/RNA UD Indexes v3, Set D, Auto (96 Indexes 96 samples)
20141199
Includes one box of 96 Illumina DNA/RNA UD v3 Indexes Set D for labeling 96 samples sufficient for automation
List Price:
Discounts:
TruSight FFPE QC Kit
20139070
TruSight™ FFPE QC Kit uses a real-time PCR assay to evaluate the quality of prospective DNA samples.
List Price:
Discounts:
Illumina DRAGEN Server v4
20051343
Includes Advance Exchange support for the first year. Requires purchase of annual DRAGEN license.
List Price:
Discounts:
ICA Basic Annual Subscription
20044874
Illumina Connected Analytics (ICA) Basic Annual Subscription. This product includes 1 year of access to ICA Basic, including sequencing instrument connectivity, data management capabilities, and access to pre-packaged analysis tools.
ICA Professional Annual Subscription
20044876
Illumina Connected Analytics (ICA) Professional Annual Subscription. This product includes 1 year of access to ICA, including sequencing instrument connectivity, data management capabilities, access to pre- packaged tools, and the ability to create customized workflows composed of tools, pipelines, data warehouses, and notebooks.
ICA Enterprise Annual Subscription
20038994
Illumina Connected Analytics (ICA) Enterprise Annual Subscription. This product includes 1 year of access to ICA Enterprise, including sequencing instrument connectivity, data management capabilities, custom and pre-packaged analysis tools, and the Base module for data warehousing and mining. ICA Enterprise also includes optional HIPAA BAA (US-only), single sign-on (SSO), and a service level agreement (SLA).
ICA Enterprise Srvc & Compliance Add-on
20066830
Illumina Connected Analytics (ICA) Compliance enables single sign-on (SSO), multi-factor authentication (MFA), HIPAA BAA (US-only), and a Service Level Agreement (SLA) for an ICA Basic Annual Subscription.
Illumina Analytics - 1 iCredit
20042038
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics Starter Pack - 1,000 iCredits
20042039
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics - 5,000 iCredits
20042040
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics - 50,000 iCredits
20042041
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Illumina Analytics - 100,000 iCredits
20042042
iCredits are used for data storage and analysis on either BaseSpace Sequence Hub or Illumina Connected Analytics.
List Price:
Discounts:
Insights - Annual Subscription
20090137
Annual subscription to Illumina Connected Insights platform.
Illumina Connected Insights – Trial Subscription
20112516
Free trial subscription for Illumina Connected Insights - for oncology.
Illumina Connected Insights – Oncology Genome Equivalent Sample - VCF
20090138
Illumina Connected Insights pre-paid oncology samples on per genome equivalent basis starting from VCF: one genome is equivalent to 2 exomes, 3 large and 6 small panel samples. Any unused samples will automatically roll over provided that the annual subscription to Illumina Connected Insights (20090137) is renewed on an annual basis. Access to a set of oncology knowledge bases is included.
TruSight Oncology 500 Training (DNA and RNA, optional HRD) - Customer Site
20031668
3.5-day, hands-on instruction at customer site to familiarize users with the essential steps in the TruSight Oncology 500 workflow. Course provides hands-on training in sample and library preparation, enrichment, sequencing, and data analysis for a maximum of four trainees.
List Price:
Discounts:
TruSight Oncology 500 Training (DNA, optional HRD) - Customer Site
20031667
Three-day, hands-on instruction at customer site to familiarize users with the essential steps in the TruSight Oncology 500 workflow. Course provides hands-on training in sample and library preparation, enrichment, sequencing, and data analysis for a maximum of four trainees.
List Price:
Discounts:
Illumina Qualified Method Training - Customer Site
20091455
This 3.5 day course is designed to familiarize new users with the automated preparation of libraries using a supported Illumina Qualified method on a Hamilton or Beckman platform through detailed hands-on instruction. Participants will complete the entire automated workflow, including library preparation, enrichment, and sequencing. Prior experience with manual preparation of the library prep kit is highly recommended.
Connected Insights Training - Remote
20092376
Illumina Connected Insights Training - Remote includes five (5) hours of product training delivered virtually.
Informatics Professional Services - Remote
20071787
Professional services for Illumina informatics products and solutions including software implementations, pipelines and workflow development, integration, networking, migration, data ingestion projects and other informatics consultative, project-based services, performed remotely, and defined by a statement of work.
TruSight Oncology 500 DNA Kit (48 samples)
20028213
Includes reagents for preparing and enriching up to 48 DNA samples and 16 indexes. Purchase NextSeq 500/550 sequencing reagents separately.
List Price:
Discounts:
TruSight Oncology 500 DNA Kit, For Use with NextSeq (48 samples)
20028214
Includes reagents for preparing and enriching up to 48 DNA samples, 16 indexes, and NextSeq 500/550 sequencing reagents.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Bundle, (16 indexes, 24 samples)
20028215
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and 16 indexes. Purchase NextSeq 500/550 sequencing reagents separately.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Bundle, for use with NextSeq (16 indexes, 24 samples)
20028216
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and 16 indexes and NextSeq 500/550 sequencing reagents.
List Price:
Discounts:
TruSight Oncology 500 DNA Kit plus Velsera interpretation report (16 indexes, 48 Samples)
20032624
Includes reagents for preparing and enriching up to 48 DNA samples, 16 indexes, and data interpretation reports using Pierian Clinical Genomics Workspace. Purchase NextSeq 500/550 sequencing reagents separately.
List Price:
Discounts:
TruSight Oncology 500 DNA Kit for Use with NextSeq plus Velsera interpretation report (16 indexes, 48 Samples)
20032625
Includes reagents for preparing and enriching up to 48 DNA samples and 16 indexes, data interpretation reports using Pierian Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Bundle plus Velsera interpretation report (16 indexes, 24 Samples)
20032626
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and 16 indexes and data interpretation reports using Pierian Clinical Genomics Workspace. Purchase NextSeq 500/550 sequencing reagents separately.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Kit for Use with NextSeq plus Velsera interpretation report (16 indexes, 24 Samples)
20032627
Includes reagents for preparing and enriching up to 24 DNA and RNA samples and 16 indexes, data interpretation reports using Pierian Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents.
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit (16 indexes, 64 Samples)
20045504
Includes reagents for preparing and enriching up to 64 DNA samples and 16 indexes. Purchase NextSeq 500/550 sequencing reagents separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit, For Use with NextSeq (16 indexes, 64 Samples)
20045505
Includes reagents for preparing and enriching up to 64 DNA samples and 16 indexes and NextSeq 500/550 sequencing reagents. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit plus Velsera interpretation report (16 indexes, 64 samples)
20045506
Includes reagents for preparing and enriching up to 64 DNA samples and 16 indexes and data interpretation reports using Pierian Clinical Genomics Workspace. Purchase NextSeq 500/550 sequencing reagents separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 DNA Automation Kit plus Velsera interpretation report, for Use with NextSeq (16 indexes, 64 samples
20045507
Includes reagents for preparing and enriching up to 64 DNA samples and 16 indexes, data interpretation reports using Pierian Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit (16 indexes, 32 Samples)
20045508
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and 16 indexes. Purchase NextSeq 500/550 sequencing reagents separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit plus Velsera interpretation report (16 indexes, 32 Samples)
20045509
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and 16 indexes and data interpretation reports using Pierian Clinical Genomics Workspace. Purchase NextSeq 500/550 sequencing reagents separately. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit, For Use with NextSeq (16 indexes, 32 Samples)
20045990
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and 16 indexes and NextSeq 500/550 sequencing reagents. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 DNA/RNA Automation Kit plus Velsera interpretation report, For Use with NextSeq (16 indexes, 32 Samples)
20045991
Includes reagents for preparing and enriching up to 32 DNA and RNA samples and 16 indexes, data interpretation reports using Pierian Clinical Genomics Workspace, and NextSeq 500/550 sequencing reagents. Intended for use with automation.
List Price:
Discounts:
TruSight Oncology 500 HRD Kit (24 samples)
20076480
TruSight Oncology 500 HRD Kit (includes HRD enrichment reagents. Does not include library prep or sequencing core reagents. HRD analysis license is required and sold separately.) Not available in Japan.
List Price:
Discounts:
TSO500 DNA/RNA Auto Kt NSQ(32) plus Illumina Connected Insights Software
20119459
Includes library prep, enrichment reagents and NextSeq reagents plus data interpretation reports (through ICI).
TSO500 DNA/RNA Kit NextSeq (24) plus Illumina Connected Insights Software
20119462
Includes library prep, enrichment reagents and NextSeq reagents plus data interpretation reports (through ICI).
Showing of
Product
Qty
Unit price
Product
Catalog ID
Quantity
Unit price
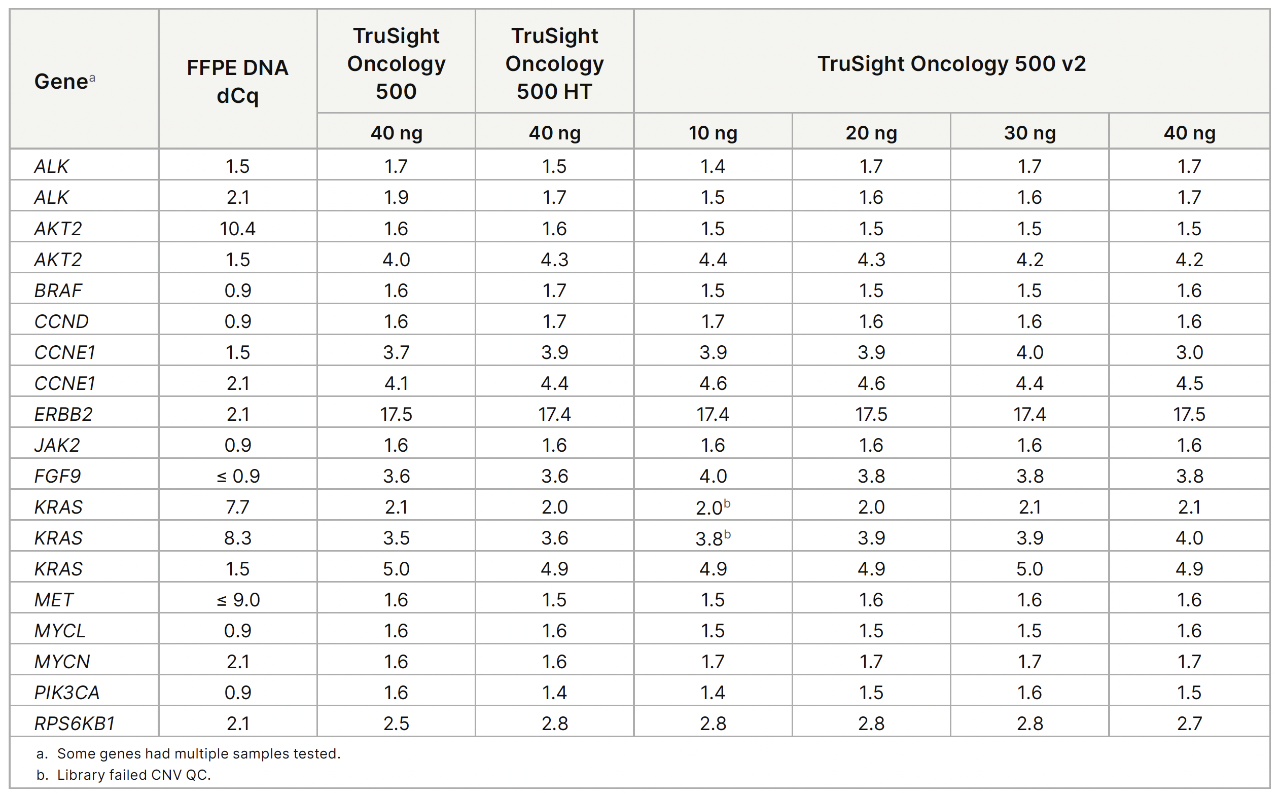
TruSight Oncology 500 v2 provides updated chemistry and a streamlined workflow that shortens turnaround time by 1–2 days. Key improvements include a single hybridization enrichment, increased DNA library coverage, better performance in GC-rich regions, more sensitive variant calling from lower input amounts, and integrated homologous recombination deficiency (HRD) analysis.
A separate high-throughput version is not needed. TruSight Oncology 500 v2 supports scalable testing with 384 unique dual indexes and broad compatibility across Illumina sequencing systems.
DRAGEN secondary analysis is required for variant calling. The DRAGEN TruSight Oncology 500 v2 analysis software is included with all TruSight Oncology 500 v2 library prep kits.
Both TruSight Oncology 500 v2 and TruSight Oncology 500 are available in manual and automated formats with third-party automation methods developed with Illumina.
Concordance data is available in the TruSight Oncology 500 v2 data sheet. Contact your local sales representative if you seek additional data beyond what is presented in the data sheet.
Complete the form to receive information about the first-time TruSight Oncology 500 v2 purchase program.
Your email address is never shared with third parties.