8 May 2023
In the very first scene of the postapocalyptic HBO show The Last of Us, a professor in the 1960s warns that if global temperatures continue to rise, certain species of fungi that were pathogenic in plants could evolve the ability to reproduce at human body temperatures. The zombie outbreak that follows is caused by a fictional variation of the real-life parasitic fungus Cordyceps—but one of the other threats that professor name-checks is Candida.
Most of the 200 species of Candida are harmless. The yeast is ubiquitous on our skin and in our gut flora. Normally our immune systems keep them from getting out of hand, but when they do—as in most vaginal yeast infections, caused by Candida albicans—we have effective antifungal drugs, usually from a group called “azoles.”
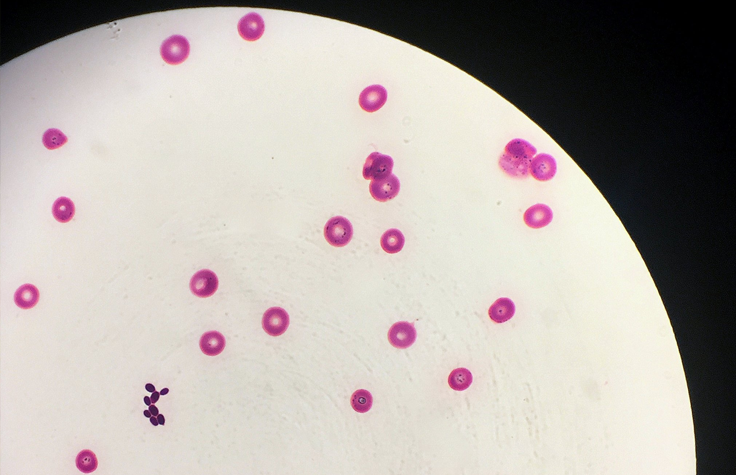
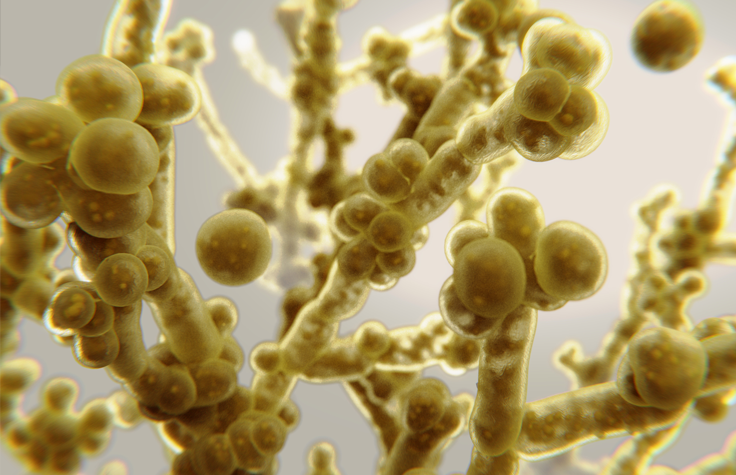
C. albicans infections have been known in humans since antiquity. However, Candida auris was first identified in 2009. Three of this species’ four known clades are resistant to all azoles, and without specialized lab equipment, it’s easy for doctors to misidentify it and fail to accurately report its spread. People with healthy immune systems can avoid becoming ill from it, but they can still carry the spores on their skin, especially in hospitals and nursing homes.
Much like with COVID-19, people who are older, immunocompromised, or have another serious illness are especially at risk from C. auris, which invades the bloodstream, nervous system, and multiple organs. By the time a patient has candidiasis, their mortality rate ranges from 30% to 60%. C. auris has spread to every inhabited continent; the Centers for Disease Control and Prevention (CDC) warns that it presents a serious global health threat. And science has yet to invent a human vaccine against fungal infection.
The closing session panel at this year’s Association of Public Health Laboratories Infectious Disease Conference focused on emerging infectious diseases. During that panel, Nevada State Health Lab Molecular Supervisor Andrew Gorzalski, PhD, reported on the biggest outbreak of C. auris in US history, which had begun in southern Nevada in 2021.
“My lab director was in the back, and he said that everyone started getting very serious looks of concern on their faces,” he recalls. “Five days after I presented, the CDC upgraded C. auris’s category to an urgent threat.”
Often, when bacteria develop mutations that make them drug resistant, their ability to grow and reproduce is limited because the proteins targeted by antibiotics are critical for cellular structure and maintenance. “The scary part with C. auris is that these mutations don’t seem to negatively affect its fitness,” Gorzalski explains. “It seems to just naturally have these resistances to first-class antifungal drugs, and they don’t impact its ability to infect people, or to live on surfaces. And through Illumina sequencing, in southern Nevada we’re seeing it start to evolve resistance to the second class in real time.”
Knowledge is power
So what can we do, in the midst of fighting one pandemic, to prevent another? The best tool we have so far is surveillance: We need to know exactly which clades of C. auris are infecting people and where, to slow the spread.
Before the pandemic, C. auris had turned up in 14 states. Two years later it had spread to 26, and that’s just the cases that have been reported to the CDC’s National Notifiable Disease Surveillance System. Despite the danger it poses, 28 states—including Nevada—still do not require hospitals to report C. auris infections. And those that do, require confirmation that the patient’s sample indeed contains C. auris and not some other Candida species.
This is where genetic sequencing proves indispensable. “We bought a lot of sequencers to handle the pandemic,” Gorzalski says, “so all of a sudden, having to sequence C. auris regularly, it’s been nice to have a NovaSeq.” The Nevada State Health Lab currently has an Illumina NovaSeq 6000, two MiniSeq Systems, two iSeq 100 Systems, and a MiSeq System. “And we plan on getting the NextSeq 1000 to really improve turnaround time.”
It goes without saying how crucial a quick response is for fighting outbreaks. “We’re getting sequencing results out faster than we’re getting susceptibility results back from our regional laboratory, so that allows us to identify those infections that are genotypically drug resistant, and then pass that information along to epidemiologists.”
With all this sequencing data, researchers can begin identifying genes that confer previously unknown mechanisms of drug resistance—and in turn, create tests that screen for those genes, detecting not only whether C. auris is present on someone’s skin, but whether that particular clade is drug resistant.
Getting the word out
The Nevada State Health Lab is facing C. auris head-on: Of the 1100 cases of C. auris in southern Nevada, the lab has sequenced 1000. “We’re basically establishing a first-of-its-kind database on this,” Gorzalski says. “Everything we sequence is put up to the National Center for Biotechnology Information. We also developed open-source bioinformatic workflows that are free to use by anyone.”
He explains that the most important thing states without current infections can do is to set up screening: Simple diagnostic PCR tests that swab from a patient’s armpit or groin can identify the fungus early on. Many states have already implemented wastewater surveillance to track the presence of COVID-19 in areas served by a particular municipal water district, or “sewershed”—this same surveillance can also detect small amounts of C. auris DNA. “This yeast can easily colonize on the skin,” he says, “so it’s going to make its way into wastewater just from people showering. One patient came from southern Nevada to another state, and immediately they could detect C. auris in the wastewater.” Scientists can trace the genetics of that tiny amount back to hospitals in that sewershed.
The more advance warning health systems have, the better prepared they can be. “We’re doing a lot more communication,” Gorzalski says, “so that other labs are more prepared for when this starts happening in their states. We’re just trying to get the information out there, because a lot of other states will be dealing with this soon.”
Intersecting outbreaks
The COVID-19 pandemic caused a raft of secondary harms for other health care concerns, and its effect on C. auris transmission is no different. “First, it actually increased exposure,” Gorzalski says; “you had more people than ever going to ICUs where C. auris already existed. Second, this was already on the radar, but suddenly all the lab testing resources were going toward COVID, and many places stopped screening for C. auris.” Although fungi spread more slowly than viruses, C. auris can live on personal protective equipment, fibrous surfaces, or asymptomatically on a person’s skin, and the disinfection techniques hospitals put in place for COVID simply aren’t effective against it. “In the lab, we use 10% bleach to kill it, but you don’t want to cover every surface of a hospital with that.”


